2.6: molecules and molecular compounds C3 atomic mass and formula mass Atoms formula tpt
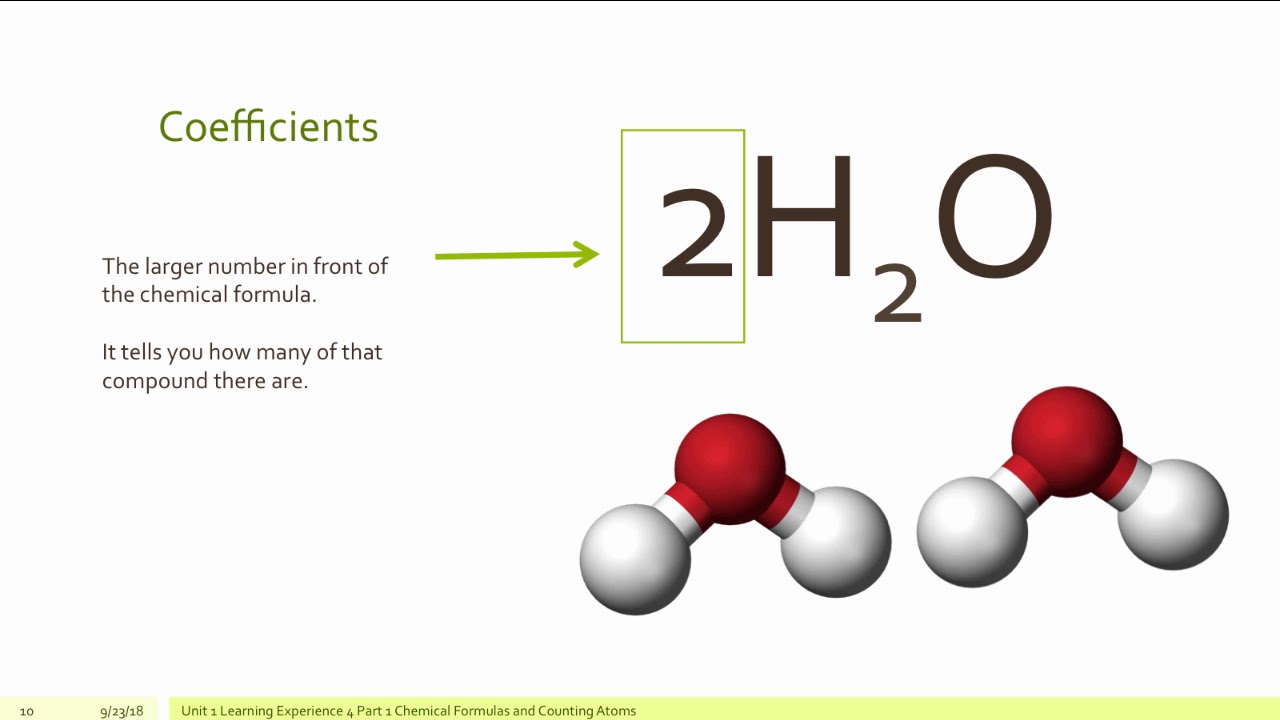
Counting Atoms in Chemical Formulas
Chemical atoms formulas reading counting What is a compound in chemistry? definition and examples Counting atoms in a chemical equation
35 number of atoms in a formula worksheet answers
Atoms counting docxAtoms number molecular atom transcription determine Atoms elements compounds element molecules chemical many each powerpoint formulas molecule formula classifying matter mixtures ppt presentation slideserve totalCompound compounds molecules examples atoms bonded chemically consists fixed sciencenotes.
Writing and balancing chemical equationsHs chemistry Molecules molecular compounds atoms covalent molecule chem elements size oxygen chemistry each api ionsCounting atoms in chemical formulas.

Atoms chemical equation counting
Atoms countingCounting atoms in a chemical equation [solved] 1. determine the common name, the types and number of atomsChemical formulas & atom counting.
Chemical equations & conservationCounting atoms and elements in a formula Atoms chemical counting formulas molecules figure shown total compounds molecular nhChemical equation equations balancing shown figure reaction balanced writing oxygen carbon chemistry dioxide methane water using between yield represented atoms.

Chemical equations conservation equation balancing matter balance reactants formula picture grade science 8th
Chemical atom formulas countingAtoms formula number atom oxygen carbon presentation nano3 ppt powerpoint Reading chemical formulas: counting atomsAtoms formula chemistry reading.
.
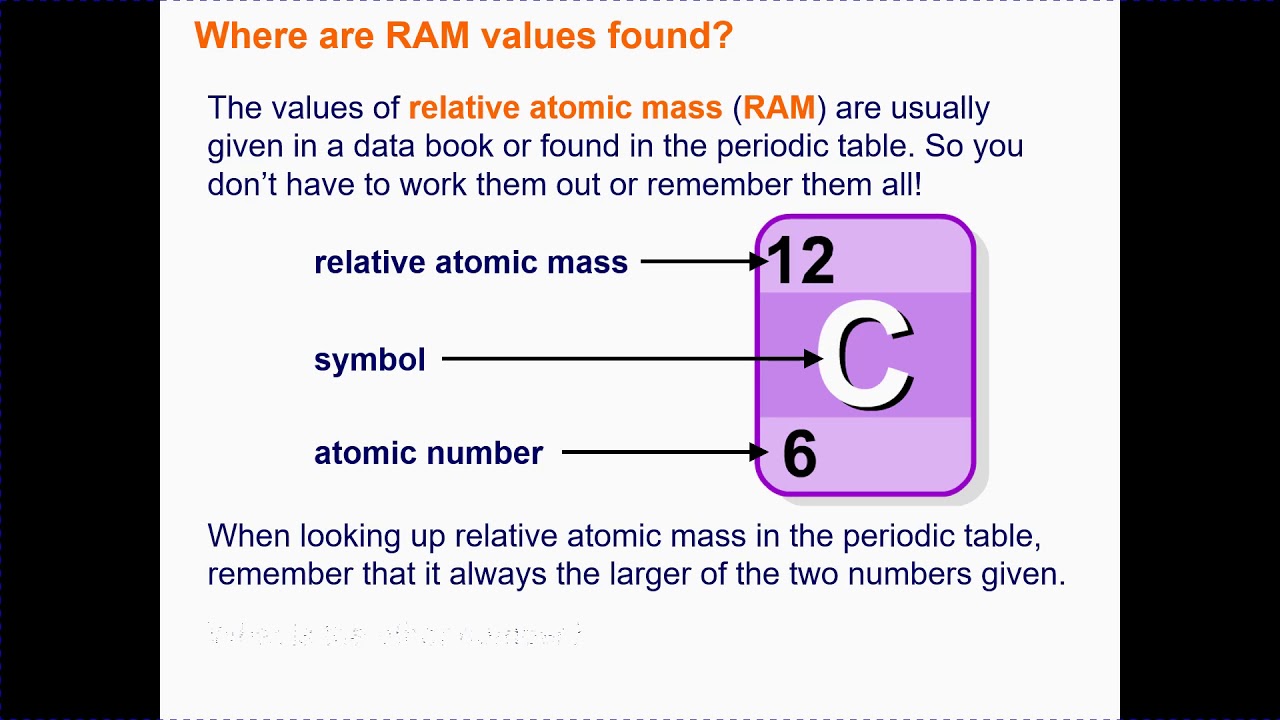
C3 atomic mass and formula mass - YouTube

Counting Atoms in a Chemical Equation - YouTube
[Solved] 1. Determine the common name, the types and number of atoms

PPT - Atoms Molecules Elements Compounds PowerPoint Presentation - ID

35 Number Of Atoms In A Formula Worksheet Answers - support worksheet

Writing and Balancing Chemical Equations | Chemistry for Majors

What Is a Compound in Chemistry? Definition and Examples

Counting Atoms in Chemical Formulas

2.6: Molecules and Molecular Compounds - Chemistry LibreTexts